3D Bioprinting has opened the generation of precisely controlled 3D cell models and tissue structures by engineering anatomically shaped substrates with tissue-like complexity.
Thanks to the high degree of structure and composition control, 3D bioprinting has the potential to solve many unmet critical needs in medical research, including applications in cosmetic testing, drug discovery, and especially in regenerative medicine and for medical research in vitro of functional organs. Custom models can be created using patient-derived stem cells such as pluripotent stem cells or mesenchymal stem cells.
Depending on the nature of the tissue to be regenerated, a specific range of materials, methods, and cells is chosen to recreate the optimal physiological conditions.
The human body has limited regenerative abilities. Current treatment options to replace damaged tissues and organs are based on obtaining tissue from the same individual or transplanting it from cadavers. There are limitations to these treatments which include donor site morbidity.
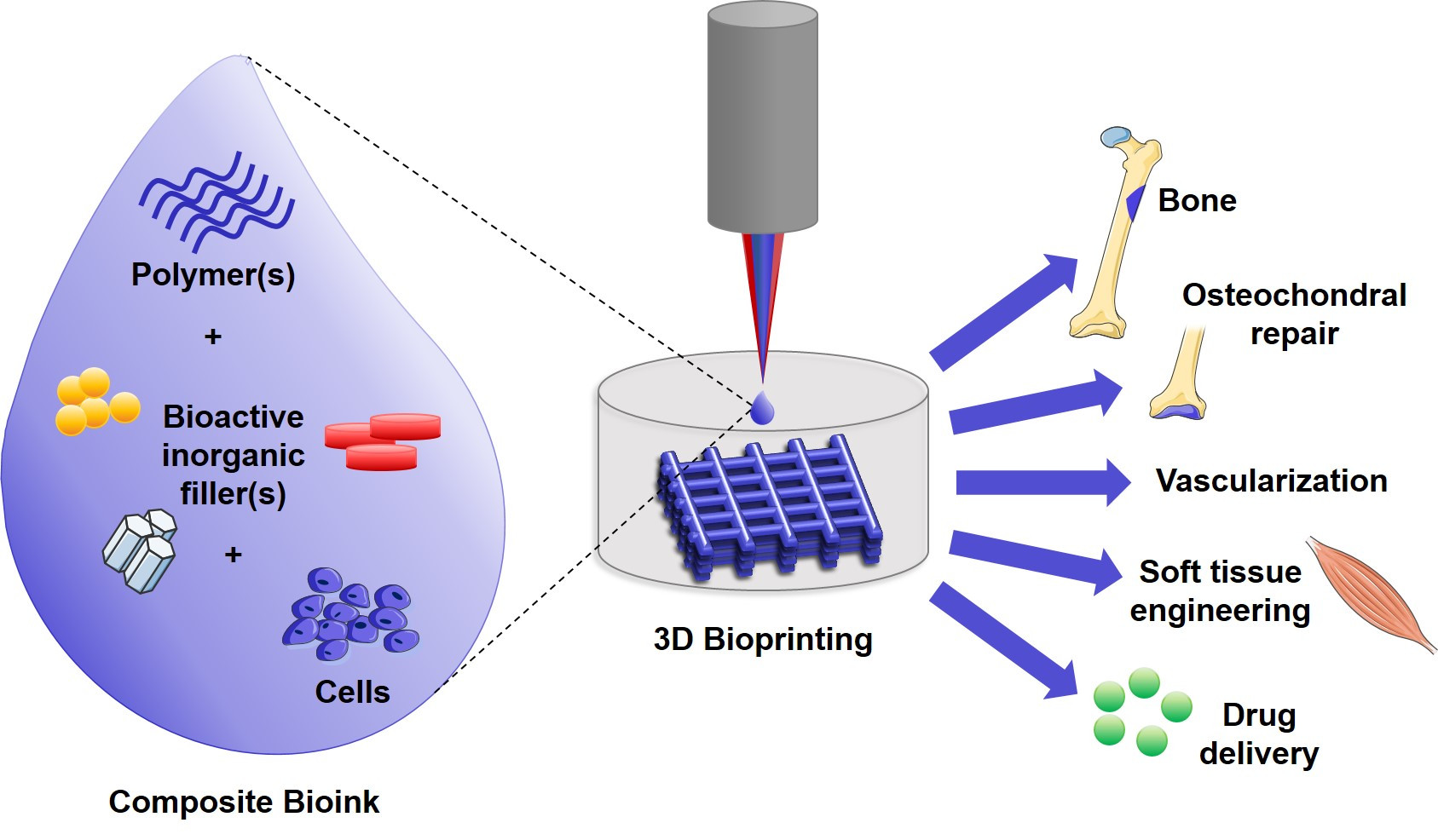
Fieldwork has evolved to create a new frontier known as regenerative engineering, defined as the convergence of advanced materials science, stem cell science, physics, developmental biology, and clinical translation towards the regeneration of complex tissues and systems of organs.
Bioprinting is an extended application of rapid prototyping or an additive manufacturing technique to print layer-by-layer (LbL) bio-functional materials. It guarantees the creation of three-dimensional cellular structures. This process overcomes the limits of traditional ones based on the study of two-dimensional cell proliferation deposited on Petri dishes because by giving the cell aggregate a three-dimensional structure, it guarantees perfect reproduction of the geometry of the organ or tissue component and physiological conditions. The process refers to printing and modeling cells or other biological entities directly onto a substrate or tissue culture dish through an automated delivery system.
The main component of the bioprinting process is the bioink consisting of living cells, growth factors, and biomaterials that mimic the environment of the extracellular matrix, favoring the adhesion, proliferation, and differentiation functions of cells after printing. A critical aspect that differentiates bioinks from traditional materials used for 3D printing are:
- Printing temperatures that do not exceed physiological temperatures
- Slight cross-linking or gelling conditions
- Non-toxic bioactive components capable of being modified by cells after printing
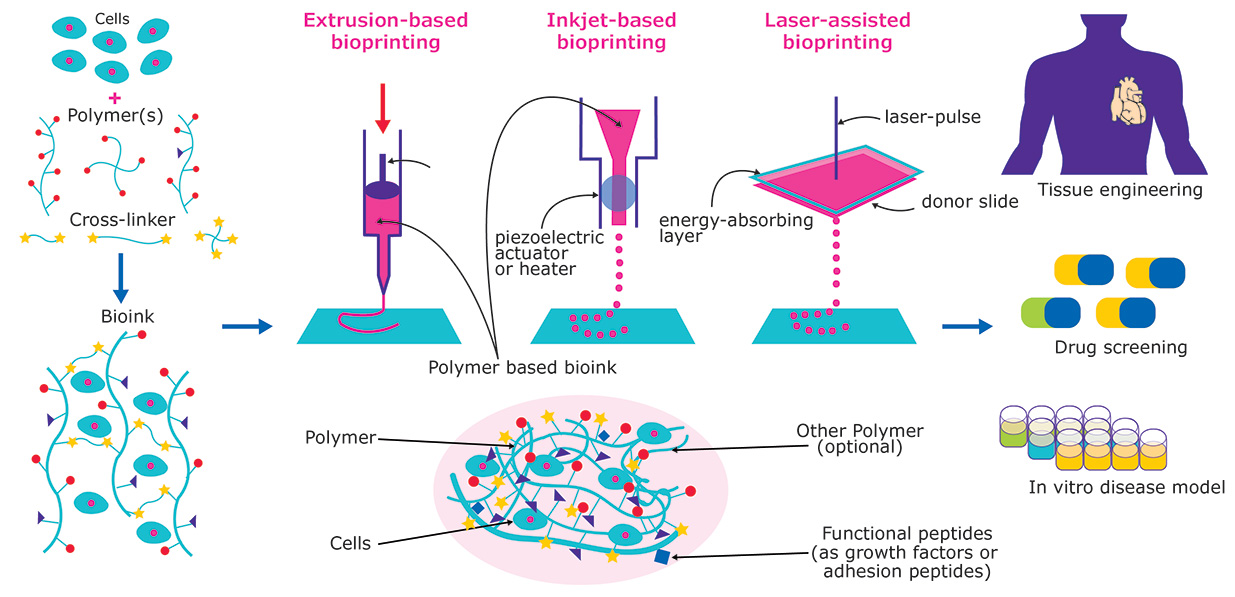
Nowadays, 3D Bioprinting consists of 3 main printing technologies that differ according to the criteria based on stimuli to assist in the deposition of bioinks:
Extrusion Bioprinting: t provides that cells suspended in prepolymer solutions are loaded into disposable medical syringes or reservoirs and subsequently printed on a platform driven by pressurized air or mechanical forces generated by a piston or rotating screw.
Inkjet Bioprinting: a non-contact technology that deposits small droplets of a bioink (1–100 picoliters) onto a receiving platform with micrometer resolution. In drop-on-demand printing, droplets with an average diameter of 10–50 μm are rapidly generated through the action of thermal or piezoelectric effects and subsequently expelled through a tiny orifice at the end of the reservoir.
Laser-assisted Bioprinting: a process that involves the application of a high-energy pulsed laser (often a near-infrared laser) on a donor tape coated with the bioink to be printed to generate the local ejection of small droplets. The incident laser light is focused primarily on a laser-transparent substrate (e.g., glass or quartz substrate), which is coated with a thin layer of metal (e.g., gold and titanium) that absorbs light energy and promotes transfer to bioink. At this time, a high-pressure bubble is generated and a small drop is pushed towards a receiving platform.