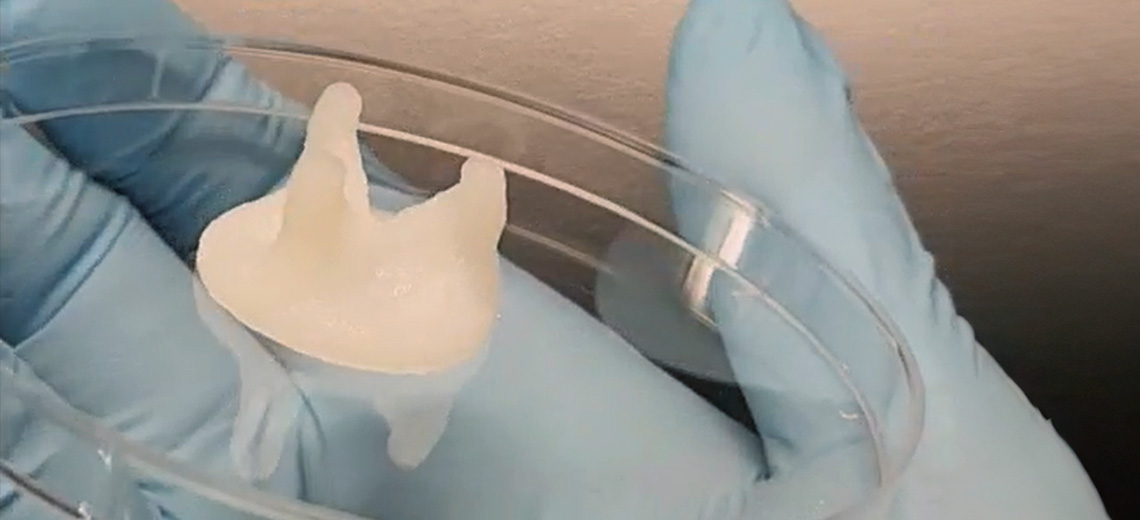
Knee menisci are macro-structurally semilunar wedges and act as a congruent cushion between the femoral condyle and tibial plateau. Meniscal tissue injuries and tears caused by sports injuries, old age and accidents are a major cause of pain and discomfort among various population groups.
These tears lead to joint destabilization and osteoarthritic developments if left untreated in the long term. Conventional treatment modalities include surgical resection of partial or total meniscus which is sometimes followed by the application of 3D meniscal implants fabricated using natural, synthetic, or hybrid materials.
The deficient healing of meniscus is due to the absence of vasculature in the mature human menisci. This could be addressed by loading the implants with the growth factors derived from the patient’s own plasma (platelet rich plasma - PRP).
A mixture of photocrosslinkable biomaterial inks including silk fibroin methacrylate (silkMA), gelatin methacrylate (gelMA), polyethylene glycol dimethacrylate (PEGDMA), lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) as photoinitiator and freeze-dried GFRP (growth factors rich plasma) in variable quantities (0, 1, 10, 25, 50 mg per ml of ink) was used for printing.

The constructs were printed using a BioX bioprinter (Cellink, Sweden) set with a temperature-controlled extruder at 18-20 °C, print bed temperature at 10 °C, a 22G blunt stainless-steel nozzle, print speed of 5-8 mm s−1, and 150-180 kPa pneumatic pressure. Furthermore, these printed structures were cross-linked using a of visible LED light (405 nm) over a span of 60 seconds to produce mechanically resilient 3D constructs that mimic the native strength of human meniscal tissue. The ink exhibited acceptable print fidelity and the fabricated construct demonstrated conformance to the desired shape.

Appropriate printing resolution and minimal distortion were observed for all biomaterials, especially the amount of GFRP reduced the buckling effect for GFRP25 and GFRP50 inks. This ensures better shape fidelity and printability of biomaterial inks.
The constructs were found to absorb approximately 3 times their dehydrated weight within 30 minutes in saline and reach equilibrium state after 16 hours, indicating efficient absorption, essential for the exchange of nutrients and metabolites. The rehydrated morphology and dimensions of the printed constructs were almost identical to their dehydrated state.
The constructs were exposed to a 20% hyperphysiological cyclic confined compressive strain. All showed a compressive modulus comparable to or greater than the equilibrium modulus of native human meniscal tissue. Typically, menisci undergo a compressive strain of 5-15% under physiological loading conditions, but even hyper-physiological strains of 20% in a cyclic manner have reported adequate outcomes, highlighting their excellent compatibility for meniscus tissue engineering applications.
Wharton's jelly-derived mesenchymal cells, hwJMSCs, were seeded onto the printed assemblies, which, when subjected to cytotoxicity assessment, showed no reduction in cell viability and were found to support cell proliferation.

Due to the significantly better biological response, the GFRP25 group was further subjected to physicochemical, mechanical and biological characterization. It demonstrated slow degradation lasting more than 6 weeks under enzymatic and saline conditions. Additionally, biological characterization of GFRP25 using hwJMSCs revealed the fibrochondrogenic differentiation competence of the constructs through upregulation of fibrochondrogenic genes and deposition of specific extracellular matrix (ECM) components of the meniscus. Cells were found to be viable and evenly distributed along the struts of the construct.
Furthermore, adequate gene expression was detected regarding aggrecan, Sox-9, collagen I and II, which were overexpressed, indicating an effective process of fibrochondrogenic differentiation of stromal cells. At the same time, a downregulation of osteogenic genes, such as runx-2 and osteocalcin, was observed, a desirable result as it suggests a lower probability of ectopic bone formation or mineralization of the graft.
It is therefore clear that these bioprinted devices allow cell proliferation, the diffusion of nutrients and resistance to hyperphysiological loads, resulting in a valid solution to problems related to lesions of the meniscal tissue.
Source: Wiley online library